BPSC 70th CCE Mains Admit Card 2025: Exam Dates Announced, Interview Call for Qualified Candidates
Candidates will need their login credentials to access the BPSC Mains admit cards.
The Bihar Public Service Commission has released the BPSC 70th CCE Mains Admit Card 2025 today, April 12, 2025. Candidates who are set to take the exam can download the admit card online by visiting either of the official websites: BPSC.bih.nic.in or bpsconline.bihar.gov.in. They will need their login credentials (application number and password) to access the admit cards online.
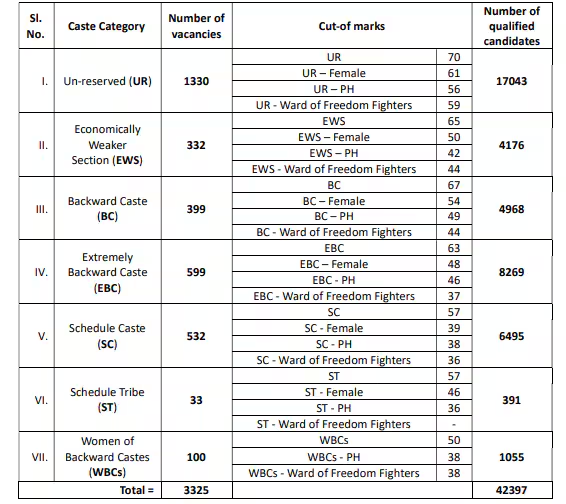
Candidates who are appearing for the Mains exam had already cleared the preliminary examination, the result of which was declared on January 23, 2025. The exam center code will be available from April 22, 2025. The BPSC 70th CCE Mains exam will be held on April 25, 26, 28, 29, and 30. Those who clear the Mains round will be called for an interview. This recruitment drive aims to fill a total of 2,035 vacancies.
BPSC 70th Mains Admit Card 2025: How To Download?
Step 1: Visit the official website of BPSC at bpsconline.bihar.gov.in.
Step 2: Click on the admit card link on the homepage.
Step 3: Enter the required credentials, such as your username and password.
Step 4: The hall ticket will be displayed on your screen.
Step 5: Download the admit card and take a printout for future use.
Candidates must carry their Bihar Public Service Commission Mains admit card to the exam center. Without the admit card, candidates will not be allowed to enter the exam hall. Ensure that you cross-check all the details on the admit card, including roll number, subjects, exam center details, and guidelines. In case of any discrepancies, candidates must report to the authorities immediately for correction.
The BPSC 70th CCE Prelims exam was held on December 13, 2024. Due to an alleged issue at the Bapu Examination Complex, Patna, a re-exam was held for over 12,000 candidates at this center on January 4, 2025. The results were declared on January 23, 2025. Of the 328,990 candidates who took the Prelims exam, 21,581 passed. Only one candidate scored a maximum of 120 marks out of 150, while 1,181 candidates scored more than 100 marks, and 6,344 candidates scored between 90 to 100 marks.