Which gas is usually liberated when an acid reacts with a metal? Illustrate with an example. How will you test for the presence of this gas?
Answer:
Hydrogen gas is usually liberated when an acid reacts with a metal.
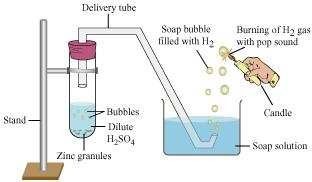
Take a few pieces of zinc granules and add 5 ml of dilute H2SO4. Shake it and pass the gas produced into a soap solution. The bubbles of the soap solution are formed. These soap bubbles contain hydrogen gas.
We can test the evolved hydrogen gas by its burning with a pop sound when a candle is brought near the soap bubbles.